PCBA
BOM
EES
Yes. We offer custom medical and healthcare electronics manufacturing solutions based on our customers’ specific needs. From custom PCBs and components to specialized assembly and testing, our EMS solutions are tailored to meet your unique project requirements. We work closely with our customers and participate in the project from the design stage to ensure that the final product meets their unique requirements and market demands.
We provide a full range of EMS solutions for the medical sector, including product design, PCB manufacturing, assembly, testing, BOM management, and after-sales support. Our services are tailored to meet the stringent requirements of medical devices and healthcare systems.
Yes. We offer comprehensive design support, including concept development, schematic design, and PCB layout, to help bring innovative medical products to the market quickly and efficiently.
Yes. We provide rapid prototyping services for medical and healthcare electronics, testing and verifying designs before moving to mass production.
Yes. We ensure compliance with international medical manufacturing regulations by operating ISO 13485-certified facilities and adhering to standards such as FDA, CE marking, and other region-specific regulatory requirements. We also support clients in achieving compliance for their devices.
Our team follows strict quality and regulatory protocols, including robust traceability systems and the use of biocompatible materials, to meet the electronic demands of the medical and healthcare industry.
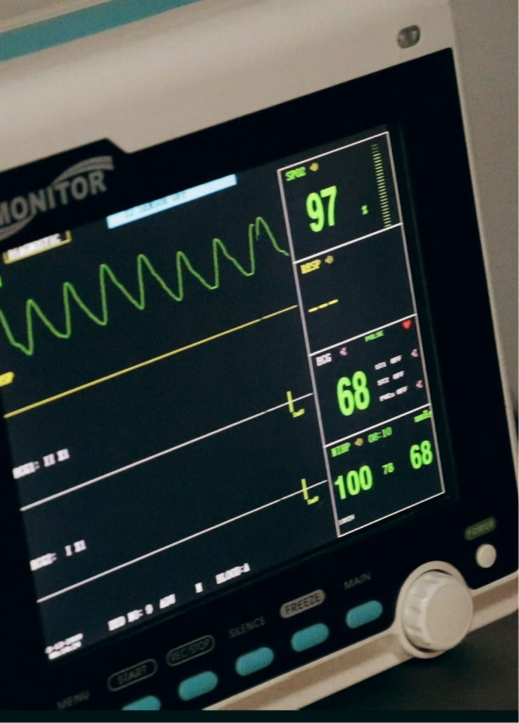
We design, build, and service complete medical systems, equipment, and products to improve efficiency and speed throughout the device’s lifecycle. This comprehensive approach ensures that our medical devices remain reliable and effective over time.
Yes, we specialize in scaling up production efficiently. Whether you require small-batch production or full-scale manufacturing, we can ensure consistent quality and timely delivery.
Yes, we offer post-manufacturing support, including maintenance, device servicing, upgrades, and lifecycle management to ensure the longevity and optimal performance of our medical devices.
The timeline depends on the complexity and scale of the project. We work closely with clients to establish realistic deadlines, ensuring timely delivery without compromising on quality.
If you are developing a new medical device, please contact us via our website or email with your design files and project requirements. Our team of experts will provide a consultation and guide you through the design, prototyping, and production phases.
To start a project with us, contact our team through our website or via email with your project details and requirements. We will provide an expert consultation to discuss your needs and outline the next steps for collaboration.
Let us run DFM/DFA analysis for you and get back to you with a report. You can send your files securely through our email at overseas@kingbrother.com.
We require the following information in order to give you a quote:
1.Gerber
2.BOM if you require assembly
3.Quantity
4.Expected price and lead time