ISO 13485 certification has emerged as the global standard for medical device quality management systems, providing a framework that ensures consistent quality, regulatory compliance, and patient safety. For medical device manufacturers, selecting a PCBA manufacturer with 13485 certificate is essential for navigating complex regulatory landscapes while maintaining the highest quality standards.
This comprehensive guide examines the critical challenges facing medical device manufacturers in PCBA production and demonstrates how ISO 13485 standards address these concerns. We’ll explore how KINGBROTHER’s ISO 13485 certification for medical devices, combined with over 28 years of manufacturing expertise, provides medical device companies with the quality assurance and regulatory compliance necessary for successful product development and market approval.
Medical device manufacturers must navigate intricate regulations across multiple jurisdictions. In the United States, the FDA requires compliance with 21 CFR Part 820 (Quality System Regulation), while European markets demand conformity with the Medical Device Regulation (MDR) 2017/745. Each framework imposes specific requirements for design controls, manufacturing processes, and quality management systems.
The challenge intensifies when devices require approval in multiple markets. A device approved for the U.S. market may need additional modifications and documentation for European CE marking. Non-compliance carries severe consequences, including product recalls, regulatory sanctions, and potential legal liability.
Medical devices demand quality levels far exceeding consumer electronics. Component failure rates acceptable in consumer products become completely unacceptable when patient safety is at stake. Medical device PCBA manufacturing requires extensive validation processes, including design validation, process validation, and ongoing quality monitoring.
Statistical process control becomes critical, as manufacturers must demonstrate that processes consistently produce products meeting specifications. The cost of quality failures extends beyond financial losses to include potential patient harm and regulatory consequences.
Medical device regulations require complete traceability from raw materials through final product delivery. Every component, process step, and quality test must be documented and traceable. In the event of a product recall or quality investigation, manufacturers must quickly identify affected products and take appropriate corrective actions.
Documentation requirements include batch records, test certificates, calibration records, and quality audit reports. Managing this documentation manually becomes impossible at scale, requiring sophisticated quality management systems and trained personnel.
Medical device PCBA manufacturing faces unique supply chain challenges. Components must meet stringent quality requirements and often require special certifications or testing. The risk of counterfeit components poses serious threats to patient safety and regulatory compliance.
Component obsolescence presents particular challenges for medical devices, which often have product lifecycles measured in decades rather than years. Supply chain qualification becomes more complex, as suppliers must demonstrate their own quality management systems and compliance with medical device regulations.
While ISO 9001 provides a general framework for quality management systems, the ISO 13485 standard specifically addresses the unique requirements of medical device manufacturing. The ISO 13485 certificate incorporates additional requirements for risk management, design controls, and regulatory compliance that are essential for medical devices.
Key differences include enhanced requirements for document control, management responsibility, and corrective and preventive actions. ISO 13485 standards also require specific processes for post-market surveillance and adverse event reporting, reflecting the ongoing responsibility manufacturers have for their products throughout their lifecycle.
ISO 13485 requirements establish comprehensive standards for quality management systems in medical device manufacturing. For PCBA manufacturers, core requirements include:
The FDA recognizes ISO 13485 certification as substantially equivalent to the Quality System Regulation (21 CFR Part 820), though some additional requirements may apply. This recognition simplifies regulatory compliance for manufacturers serving both U.S. and international markets.
European regulations explicitly reference ISO 13485 certification for medical devices as the harmonized standard for medical device quality management systems. Other major markets, including Canada, Australia, and Japan, recognize the 13485 certificate, making it a global passport for medical device market access.
ISO 13485 certification ensures PCBA manufacturers implement systematic approaches to quality management that prioritize patient safety. The standard requires risk-based thinking throughout all processes, ensuring potential safety issues are identified and addressed before they can impact patients.
Preventive action requirements help identify and eliminate potential quality problems before they occur. This proactive approach significantly reduces the risk of product recalls and patient safety incidents while ensuring continuous improvement processes that evolve quality management systems over time.
ISO 13485 standards facilitate market access across multiple jurisdictions. Medical device manufacturers can leverage their supplier’s ISO 13485 certification to support their own regulatory submissions and quality system documentation.
Regulatory authorities worldwide recognize the ISO 13485 certificate as evidence of effective quality management systems. This recognition can expedite regulatory reviews and reduce the burden of additional quality system assessments, supporting global supply chain strategies regardless of geographic location.
ISO 13485 requirements mandate comprehensive risk management processes throughout the product lifecycle. For PCBA manufacturing, this includes identifying and controlling risks related to component selection, manufacturing processes, and quality testing.
Statistical process control requirements ensure manufacturing processes remain in control and capable of meeting specifications. Corrective and preventive action systems ensure quality issues are thoroughly investigated and permanently resolved through root cause analysis.
FDA recognition of ISO 13485 standards can simplify regulatory submissions and inspections. A PCBA manufacturer with 13485 certificate can reference this certification in their quality system documentation and regulatory submissions.
The systematic approach required by ISO 13485 certification for medical devices aligns well with FDA expectations for quality systems, reducing regulatory risk and supporting successful FDA inspections and audits.
KINGBROTHER’s ISO 13485 certification demonstrates our commitment to maintaining the highest quality standards for medical device manufacturing. Our quality management system incorporates all ISO 13485 requirements, including design controls, risk management, and regulatory compliance processes.
Our certification is maintained through regular surveillance audits and continuous improvement initiatives, ensuring our quality management system remains current with evolving regulations and industry best practices. Management’s commitment to quality extends throughout our organization, from senior leadership to manufacturing floor personnel.
KINGBROTHER maintains qualified supplier networks specifically for medical device applications. Our component sourcing processes include verification of supplier quality systems, component testing and validation, and ongoing supplier monitoring to ensure continued compliance.
We implement comprehensive material control processes, including incoming inspection, material identification and traceability, and controlled storage conditions. Component obsolescence management programs help medical device manufacturers plan for long-term product support with maintained component roadmaps and alternative sourcing strategies.
Our testing capabilities include comprehensive electrical testing, environmental testing, and reliability validation appropriate for medical device applications. All testing equipment is calibrated according to ISO 13485 standards and traceable to national standards.
Statistical process control implementation ensures our manufacturing processes consistently meet specifications. We collect and analyze process data to identify trends and implement preventive actions before quality issues can occur, providing comprehensive test documentation and certificates of conformance that meet medical device industry standards.
KINGBROTHER operates controlled manufacturing environments appropriate for medical device assembly. Our clean room facilities meet appropriate cleanliness standards and include environmental monitoring and control systems.
Personnel training programs ensure all staff understand medical device quality requirements and their role in maintaining product quality. Contamination control procedures protect products throughout the manufacturing process, including material handling protocols, equipment cleaning procedures, and environmental monitoring programs.
Our quality management system provides complete traceability from component receipt through product shipment. Batch records document all manufacturing activities and test results, supporting regulatory compliance and product recalls if necessary.
Document control processes ensure all quality-related documents are current, accurate, and accessible. Electronic quality management systems support efficient data collection and reporting, providing real-time visibility into quality metrics and rapid response to quality issues.
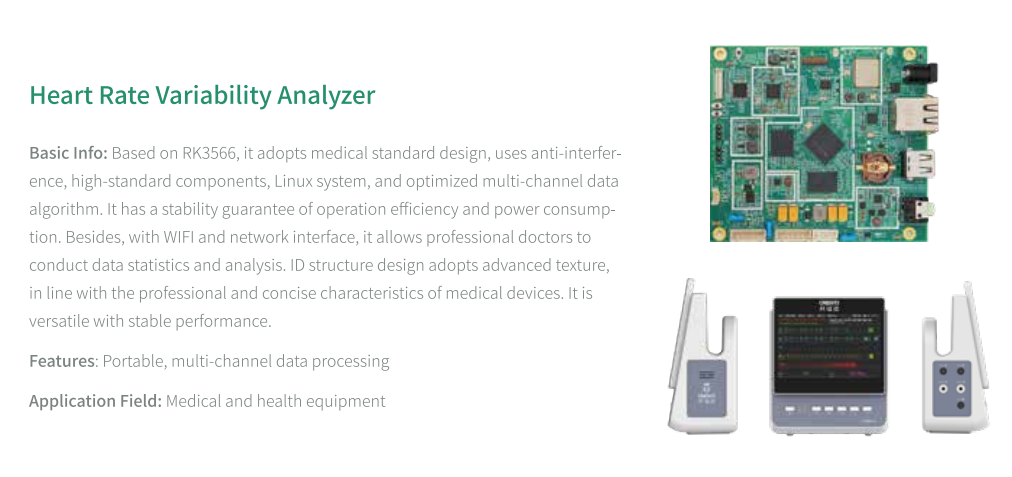
KINGBROTHER supports manufacturers of diagnostic equipment, including blood analyzers, chemistry analyzers, and point-of-care testing devices. These applications require high-precision electronics capable of accurate measurement and reliable operation in clinical environments.
Our experience includes PCBAs for laboratory automation systems, requiring integration of multiple sensors and actuators with sophisticated control electronics. Quality control processes for diagnostic equipment focus on measurement accuracy and repeatability through statistical process control and validation procedures.
Patient monitoring applications require exceptionally reliable electronics, as failure can directly impact patient safety. KINGBROTHER’s ISO 13485 certification ensures patient monitoring PCBAs meet the highest reliability standards.
Our experience includes vital signs monitors, cardiac monitors, and continuous monitoring systems. These applications require careful attention to signal integrity, electromagnetic compatibility, and patient safety isolation requirements. Reliability testing includes accelerated life testing and failure mode analysis.
Medical imaging applications demand high-performance electronics capable of processing large amounts of data with minimal noise and distortion. KINGBROTHER’s advanced manufacturing capabilities support the complex PCBAs required for imaging applications.
Our experience includes PCBAs for ultrasound systems, X-ray equipment, and MRI systems. These applications require careful attention to signal integrity, electromagnetic shielding, and thermal management. High-frequency design capabilities support advanced imaging applications requiring sophisticated signal processing.
Therapeutic and surgical devices often require custom PCBAs designed for specific applications and environments. KINGBROTHER’s design and manufacturing capabilities support the development of specialized electronics for these demanding applications.
Our experience includes PCBAs for surgical robots, therapy delivery systems, and implantable devices. These applications require exceptional reliability and often involve unique environmental requirements. Biocompatibility considerations for implantable devices require special attention to material selection and manufacturing processes.
KINGBROTHER’s ISO 13485 certification for medical devices, combined with over 28 years of manufacturing expertise, provides medical device manufacturers with the quality assurance and regulatory compliance necessary for successful product development and market approval.

Our commitment to quality extends beyond certification to include continuous improvement, advanced manufacturing capabilities, and comprehensive customer support that ensures medical device success through reliable partnerships focused on patient safety and regulatory compliance.