Printed Circuit Boards (PCBs) play a crucial role in the sleek and efficient medical devices we rely on every day.
They form the core of electronic gadgets, simplifying complex circuits and ensuring consistent performance. The process of creating a PCB involves meticulously layering conductive paths and assembling them to comply with strict medical industry standards.
Their importance within the healthcare industry has grown substantially. As technology continues to advance rapidly, its role in medical devices is expected to expand further, driving innovation and enhancing patient care.
In the context of the medical industry, PCBs are electronic boards specially designed to meet stringent industrial requirements. They help to keep a wide range of medical devices running, such as diagnostic equipment, monitoring systems, and implantable devices.
They are precise and reliable, both of which are qualities necessary for medical applications that may impact a patient’s health and safety.
Thus, the manufacturing of medical PCBs is highly scrutinized and regulated to ensure only the highest quality products reach the market.
PCBs play a vital role in today’s healthcare by ensuring medical equipment operates reliably and accurately. Even minor mistakes can endanger patient safety, so PCBs create a solid foundation for intricate circuits, ensuring devices work as intended.
Medical PCBs are distinguished by their compact size, high performance, and durability. They are designed to be both small and powerful, making them ideal for portable medical devices.
Despite their size, they can manage demanding tasks and maintain reliability over time, even in challenging medical settings.
Moreover, PCBs incorporate advanced technologies like the Internet of Things (IoT) and Artificial Intelligence (AI).
IoT allows for real-time health monitoring and data collection, enhancing patient care by tracking health conditions and enabling remote diagnostics.
AI supports healthcare professionals by analyzing data, predicting conditions, and providing customized treatment options.
Suggested reading: How to Make a PCB: PCB Manufacturing Process Guide
PCBs are a vital component of the healthcare industry, significantly improving the performance and reliability of medical devices. They play a critical role in ensuring accurate and precise diagnostics and treatments, which are essential for delivering high-quality patient care.
Designed to meet strict safety standards, PCBs prioritize patient safety while complying with regulatory requirements.
High-quality PCBs play a critical role in ensuring stable signal transmission and superior signal recognition capabilities in medical electronic devices. These advanced features help minimize interference and optimize signal clarity, which is essential for improving the accuracy of medical equipment. For example, in diagnostic imaging systems like MRI or CT scanners, high-quality PCBs enable precise image rendering, providing detailed visuals for accurate diagnoses. Similarly, enhanced signal recognition supports advanced diagnostic tools in offering reliable guidance during procedures, ultimately boosting patient outcomes and medical precision.
Additionally, PCBs can be customized for specific medical processes, facilitating technological advancements and supporting the development of innovative medical devices to address the evolving needs of the healthcare industry.
Furthermore, PCBs contribute to the miniaturization of medical devices, enabling the creation of compact and portable equipment that enhances patient convenience and accessibility.
They also play a key role in integrating medical devices with IoT networks, paving the way for smart healthcare solutions such as real-time monitoring and remote care.

The following are the key types of medical PCBs:
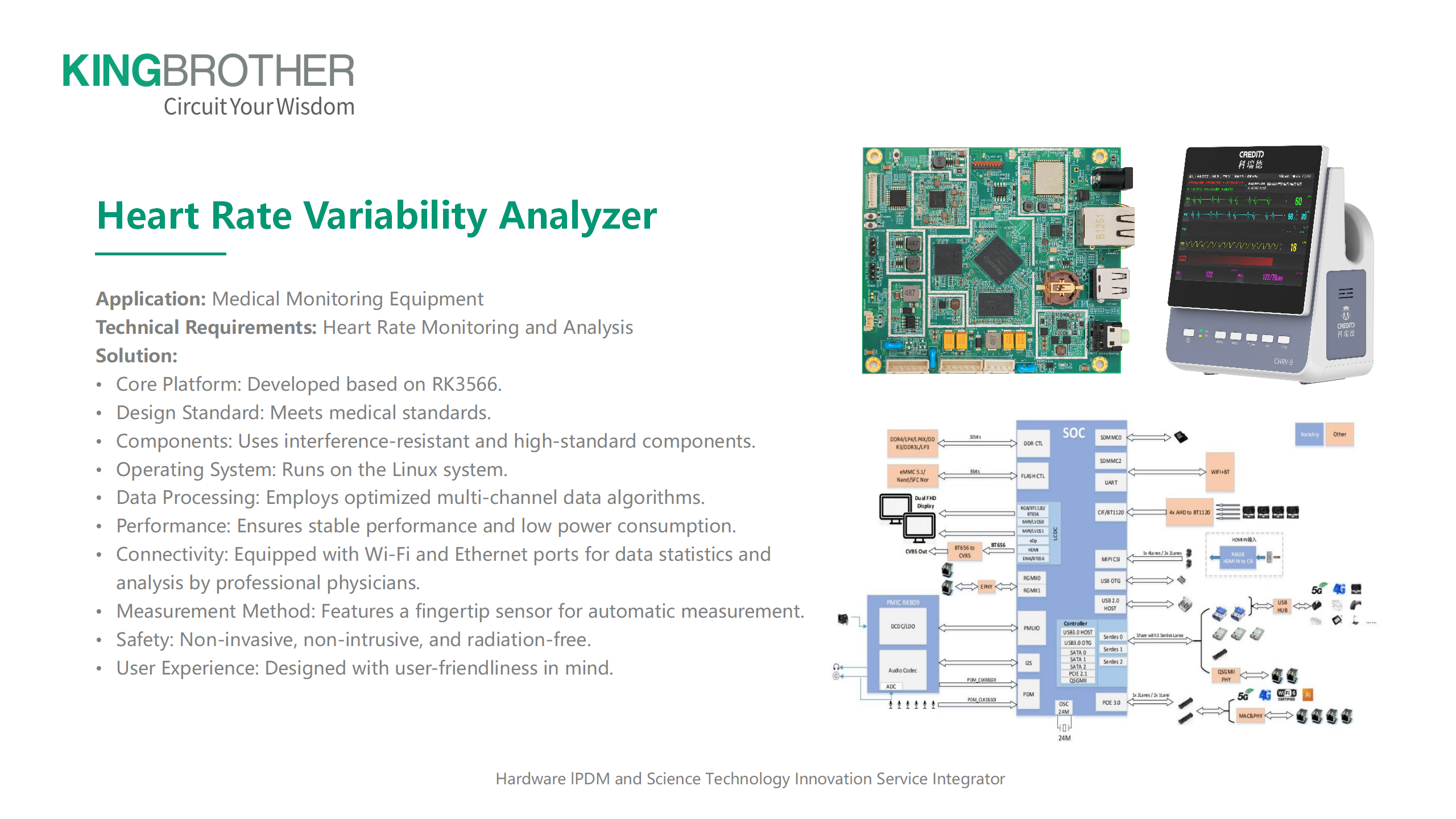
The production of PCBs for medical devices is a complex process that must follow strict standards to ensure quality, safety, and reliability. There are many guidelines to control PCB production and ensure that these important products meet the highest safety and quality standards. The main criteria and requirements are:
These regulations govern the approval process for medical-grade devices. They recognize that PCBs are safe, effective, and suitable for medical use. This regulatory approval is required to enter the market and sell products.
These standards set performance standards for reliability for critical applications. They ensure that PCBs meet the highest quality and reliability standards at all times. Additionally, it keeps medical devices dependable and durable even after years of use.
Manufacturing printed circuit boards (PCBs) for medical devices comes with important challenges that must be addressed to ensure top quality, safety, and performance:
Biocompatibility and Safety in Implantable Medical Devices
A key challenge is ensuring that implantable devices are both safe and compatible with the human body. The materials used in PCBs for these devices must be non-toxic and harmless to human tissues, requiring thorough testing and strict adherence to biocompatibility standards to protect patients.
Optimizing for Size and Performance
Another challenge involves designing smaller devices without sacrificing performance. As medical devices shrink in size, PCBs need to fit into tight spaces while still being functional and reliable. This often calls for creative design solutions and advanced manufacturing methods.
Complying with Stringent Regulatory and Quality Standards
Compliance with strict regulatory and quality standards is also critical. Medical PCBs must meet various aforementioned international requirements, such as ISO 13485, IEC 60601, and regulations from the FDA and EU. This requires careful documentation, quality control, and validation throughout the production process.
Improving The Efficiency of Product Development and Testing
Long product development and testing cycles also pose a significant hurdle. Creating and testing medical PCBs involves extensive validation to meet performance and safety criteria, which can extend the timeline and demand considerable resources and time.
Ensuring Longevity in Challenging Conditions
Ensuring reliability and durability is vital, especially in challenging conditions. Medical PCBs must operate consistently in environments exposed to moisture, chemicals, and extreme temperatures. This depends on using high-quality materials and strong design practices for long-lasting performance.
Integrating Advanced Technologies into Design and Manufacturing
Finally, adding advanced technologies increases the complexity of PCB design and manufacturing. As medical devices integrate features like high-speed data transmission, wireless connectivity, and improved signal processing, the PCBs must remain compact while staying reliable.
Recent improvements in PCB design and manufacturing for the medical field have greatly advanced healthcare technology.
New materials like biocompatible polymers and ceramics are now creating lighter and sturdier PCBs for implantable and wearable devices. These enhance the durability and dependability of medical devices while ensuring safety for long-term use within the body.
The microelectronics field has also contributed by allowing smaller, more efficient components in PCB designs. This miniaturization means medical devices can be more compact and user-friendly, making them easier for patients to handle. Smaller components enable devices to offer more features in a limited space, enhancing diagnostic and treatment capabilities.
Additionally, integration with IoT technology is transforming medical devices into smart systems that can send data in real time and allow for remote monitoring. Devices can continuously gather and share patient data with healthcare providers, enabling prompt treatments and personalized care.
Finally, sustainable practices in PCB manufacturing are gaining traction, with many companies adopting eco-friendly processes to lower their environmental impact.
More manufacturers are using lead-free solder, recyclable materials, and energy-efficient production techniques. These approaches improve device safety and effectiveness while showing a commitment to protecting the environment.
Working with a medical device PCB manufacturer can net you many benefits.
Among these, compliance with medical industry standards guarantees that PCBs meet stringent requirements, ensuring safety and reliability.
Collaborative design and development have the added benefit of sharing knowledge and driving innovation and improvement. This process allows for extensive and effective testing prior to full production, reducing the risk of errors.
This relationship is necessary for medical devices to be dependable. Extensive testing is performed to ensure that the PCB meets all performance and safety standards. This further helps protect patient health by identifying and eliminating potential problems before faulty PCBs are used in medical devices.
In conclusion, PCBs are the backbone of modern medical devices, offering precision, reliability, and adaptability to meet the stringent demands of the healthcare industry.
From enabling real-time monitoring to powering compact, high-performance equipment, PCBs drive innovation and elevate patient care. As technology continues to evolve, the role of PCBs will only grow, shaping the future of medical advancements and sustainable healthcare solutions.
Partnering with a trusted PCB manufacturer like Kingbrother provides valuable experience, teamwork, high-quality production, and thorough testing. These factors are essential for creating dependable and advanced medical devices that can transform healthcare. For more information and to explore our range of solutions, visit our website at en.kingbrother.com. Let us help you turn your medical device ideas into reality with PCBs designed for excellence.